Cardiovascular diseases associated with limited functioning of the heart are reported to cause the highest rate of mortality, with the risk factors of aging, smoking, overweight, and inadequate exercise in both industrialized and developing countries. Such factors can lead to mechanical stimuli in the form of pressure overload and conditions can progressively result in irreversible defects on the heart. In addition, more than 17 million people are suffering from heart failure worldwide, and over 50% of these patients do not effectively respond to current pharmacological therapies. Therefore, heart-related dysfunctions remain a significant clinical challenge. Surgical procedures, angioplasty, valve reconstruction/replacement, and heart transplantation have been clinically utilized to address this challenge, however, with limited success only. When medical and surgical treatments are not optimal in patients with heart failure and coronary artery diseases, cardiac transplant remains the only solution. However, heart-transplanted individuals face lifelong immunosuppression, at the expense of hypertension, diabetes, and renal failure. Thus, novel and personalized approaches must be developed for heart diseases to compensate for complications associated with current clinical strategies.
At BIREL, we aim at developing procedures for decellularized ECM scaffolds and evaluating decellularization efficiency in terms of residual nuclear content and structural properties. We expect that the bioartificial scaffolds formed can be functionalized with patient’s own material and utilized in regenerative engineering.
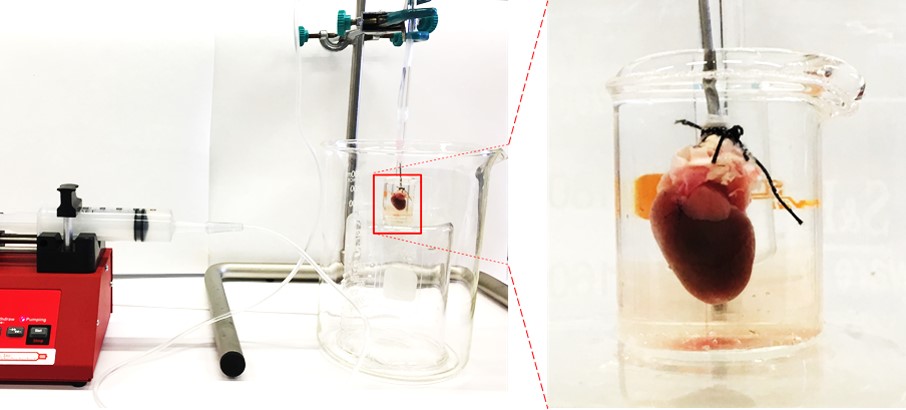

Experimental set-up for the decellularization process: (a) general view of the setup and (b) the magnified portion of the cannulated heart.
Decellularization of the rat heart. (a) Photographs showing each step of the first cycle of the decellularization process: (0) before decellularization, and after (1) deionized water, (2) PBS, (3) enzymatic (trypsin) solution, (4) 1% SDS solution, (5) 3% Triton X-100 solution, (6) deoxycholic acid, (7) peracetic acid, (8) PBS, (9) deionized water, (10) PBS, and (11) deionized water perfusion. (b) Photographs showing each step of the second cycle of the decellularization process after (1) deionized water, (2) PBS, (3) enzymatic solution, (4) 1% SDS solution, (5) 3% Triton X-100 solution, (6) deoxycholic acid, (7) peracetic acid, (8) PBS, and (9) deionized water perfusion. Scale bar is 2.54 cm.
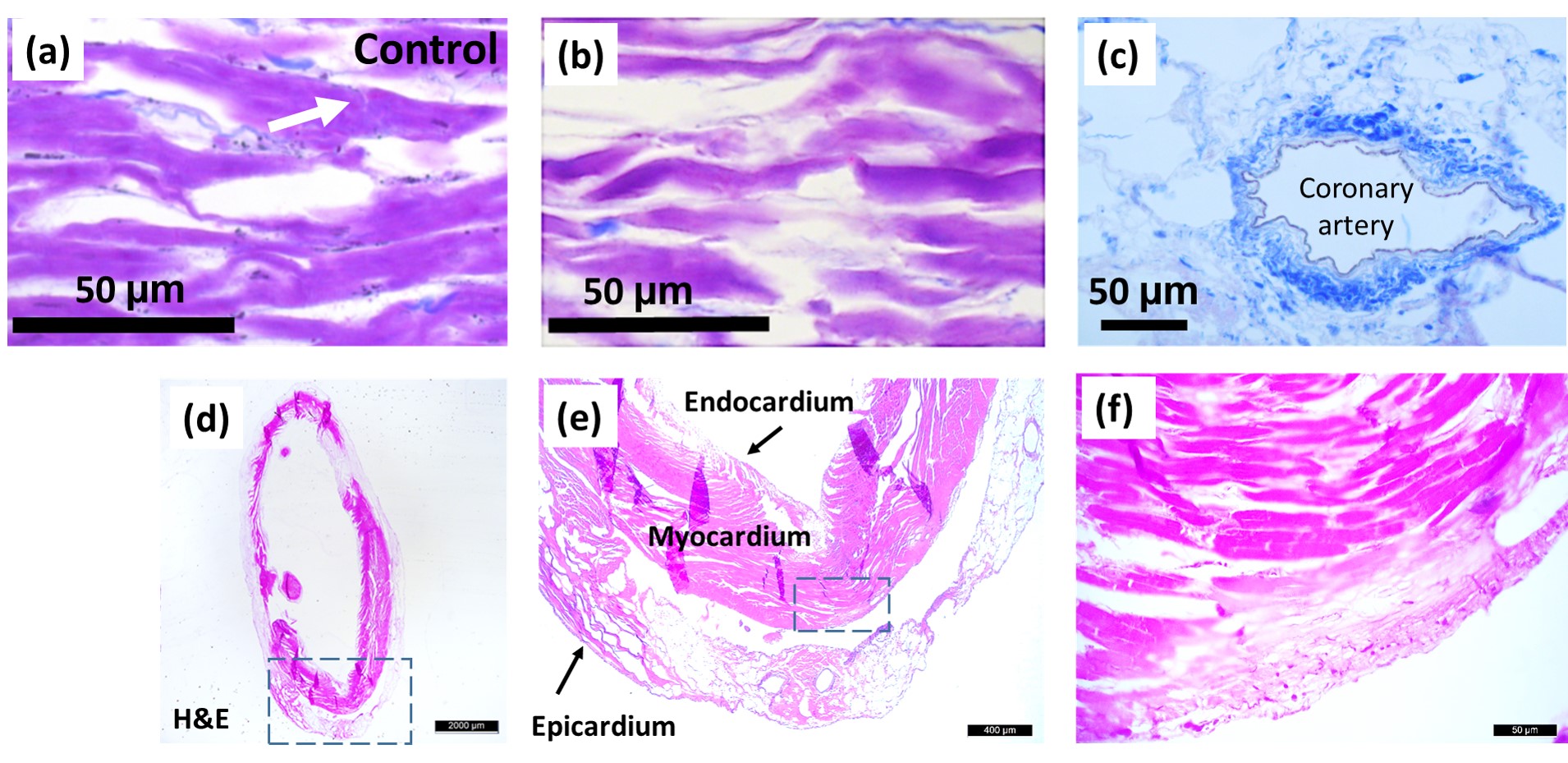
Crossman’s modified triple-stained sections from cadaveric rat hearts of the control group (a) and decellularized group (b, c). Connective tissue is dyed with aniline blue (blue). Longitudinal cardiac muscle fibers formed by interconnected cardiomyocytes of (a) native and (b) decellularized hearts. Intercalated disk is pointed by white arrow in a. Stains of decellularized rat heart (d–f). (e, f) Magnified portions of d and e, respectively, indicated by the rectangle in dashed lines. Scale bar is 2 mm in d, 400 μm in e, and 50 μm in f.
Source: Ozlu et al., The International Journal of Artificial Organs 2019, Vol. 42(12) 757–764

